Topics Map > Standard Operating Procedures (SOP)
Controlled Substance Discrepancy Resolution SOP
VTH SOP # SOP-005-CS
Category: Controlled Substances
Number: SOP-005-CS
Date of Last Review: January 26, 2026
Next Review Date: January 2028
Printable PDF:
Purpose/ Applicability:
To describe the process of investigating, resolving, and documenting controlled substance discrepancies.
Scope:
This applies to all controlled substance discrepancies identified within the VTH. This does not apply to MDVC.
Associated Policy: Controlled Substance Discrepancy Resolution Policy
Procedure:
Investigating Controlled Substance Discrepancies
When a controlled substance discrepancy is identified, the following steps should be followed to determine the cause.
- Verify the accuracy of the discrepancy through a double check of the quantity on hand to verify that, after the discrepancy adjustment, the quantity on hand is actually correct.
- If the quantity on hand is still incorrect, make the necessary adjustment and repeat the verification. Ideally, the verification will involve a second person so that it is not being verified by the same person who created the original discrepancy.
- If the drug is a multi-dose vial, and the discrepancy was an increase in the quantity on hand, determine if the amount could reasonably be due to manufacturer overfill. The allowed amount of overfill per vial is 10% of the labeled quantity. For example, a 20ml vial may have up to 2 ml of overfill. Past trends should be used to determine whether overfill is reasonable for a given drug, as the actual overfill amount varies by drug and manufacturer.
- If the drug is a multi-dose vial/bottle, and the discrepancy was a decrease in the quantity on hand, count the number of sticks/draws since the last known actual draw or opening of a new vial/bottle. Multiply the number of sticks by 0.05 for injectable medications and by 0.25 for oral liquids to determine the volume that can be accounted for by hub loss. If the calculated hub loss is greater than or equal to the discrepancy amount, the discrepancy can be resolved as a result of hub loss.
- For Cubex records, count the discrepancy transaction as ‘1’ and then count each dispensation transaction in that bin as an additional stick until the next discrepancy.
- For paper logs, count the discrepancy draw as ‘1’ and then count each logged dispensation between the last actual draw and the current discrepancy.
- If hub loss is close, but doesn’t quite cover the entire discrepancy, recalculate hub loss using a value of 0.06ml/stick for injectable medications and 0.30ml/draw for oral medications (compounded oral suspensions may allow 0.40mL/stick hub loss, based on previous testing and evidence). If that covers the discrepancy, it can be resolved with a note of ‘WATCHING’ to indicate that increased oversight is necessary in the immediate future until a trend is identified or ruled out.
- The VTH Controlled Substance Surveillance Program Manager shall provide a report to the VTH DEA Registrant of any drugs that have a designation of ‘WATCHING’ and the increased monitoring that is taking place. The report should also indicate any drugs previously under increased monitoring that have been removed from that monitoring.
- For WMC drugs marked at ‘WATCHING’, the WMC Director should also be notified.
- The VTH Controlled Substance Surveillance Program Manager shall provide a report to the VTH DEA Registrant of any drugs that have a designation of ‘WATCHING’ and the increased monitoring that is taking place. The report should also indicate any drugs previously under increased monitoring that have been removed from that monitoring.
- If the drug is not a multi-dose vial/bottle or the discrepancy cannot be accounted for by overfill or hub loss alone, review all logged transactions since the last known actual count to identify any problematic transactions. If any are identified, investigate to determine the actual amount dispensed and whether this accounts for the discrepancy. Examples of problematic transactions are the following:
- A Cubex timeout (TO) transaction
- A logged quantity that is different than the previous quantities removed for that patient.
- A logged quantity that deviates from the calculated dosage range for the patient.
- Any Cubex transaction for a quantity of zero.
- A skipped Cubex transaction.
- Multiple dispensations for the same patient and drug over an unusually short period of time.
- Doses documented in the patient chart that don’t match a transaction logged in Cubex in regards to quantity, drug, date, and time.
- Administrative transactions (stocking, unstocking, cycle counting, etc.) that do not follow typical trends/expectations.
- If the drug in question is ever used for filling prescriptions entered in the HIS and the discrepancy cannot be resolved with the previous steps, determine the last date where the exact quantity on hand was known to be accurate (usually the last discrepancy).
- Pull all prescription records from the HIS from the last known accurate count until the current discrepancy. Ensure that each prescription entered in the HIS has a corresponding Cubex transaction. Transactions in Cubex should match the date, drug, quantity, and patient name to the prescription entered in the HIS.
- If there are Cubex transactions that don’t have a corresponding HIS prescription, determine if the patient was charged appropriately and enter an HIS transaction if needed.
- If there are HIS prescriptions that don’t have a corresponding Cubex transaction, determine if the prescription was actually filled. If filled and not logged in Cubex, this quantity should be counted towards resolving the discrepancy.
- Determine if the missing Cubex transaction was the result of a process error or a Cubex glitch. If caused by a process error, address the error with those involved. If caused by a Cubex glitch, contact Cubex support to report the glitch and have it investigated.
- Pull all prescription records from the HIS from the last known accurate count until the current discrepancy. Ensure that each prescription entered in the HIS has a corresponding Cubex transaction. Transactions in Cubex should match the date, drug, quantity, and patient name to the prescription entered in the HIS.
- If the previous steps did not account for the full discrepancy amount, track all drug movement to determine if a stocking error is the cause of the discrepancy.
- Determine the last date when the drug amount was known to be correct in the bin in question.
- Document each Cubex machine holding the drug in question and the amount on hand in each as of the date determined in (a). This will be referred to as the ‘starting balance’.
- Complete a cycle count of each of these bins as of the date of the discrepancy to ensure all other bins containing the drug in question show correct amounts.
- Document the amount on hand in each Cubex machine based on the cycle counts performed in (c). This will be referred to as the ‘ending balance’.
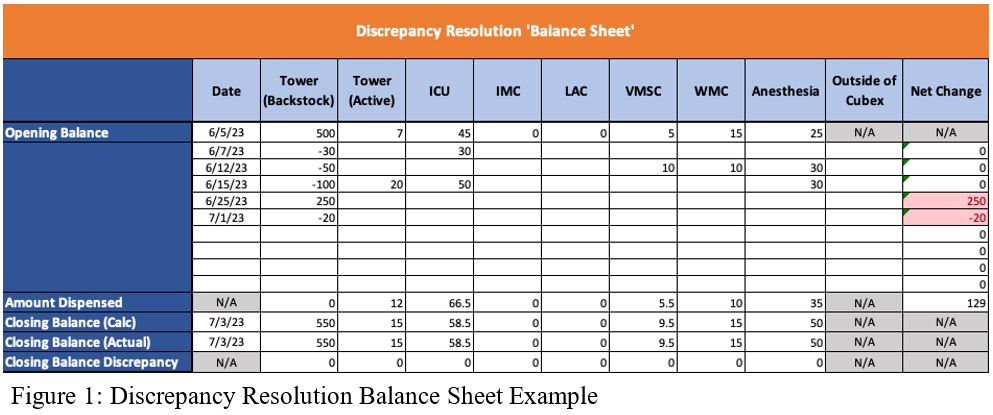
- Pull all stocking and unstocking transactions for that drug across all Cubex machines from the starting balance through the ending balance. Log these transactions in a balance sheet to follow the drug between machines. See Figure 1 for an example Discrepancy Resolution Balance Sheet. This step is based on the following assumptions.
- All controlled substances that are received are stocked into the Medication Dispensary’s Tower Cabinet immediately after receipt. Verify the quantity of each of these stocks aligns with a packing slip indicating that amount was received on that date.
- All controlled substances in any Cubex besides the Tower are obtained from the Tower stock. For example, a stock of 10ml in the ICU Cubex will have a corresponding unstock transaction for 10ml in the Tower Cubex.
- A drug that is removed from the Cubex system (e.g., for research use or ambulatory bags) has a paper trail. Verify each of these through the yellow sheets and the drug signed out.
- If completing the first five steps doesn’t fully resolve the discrepancy, access camera footage to watch each transaction that may have contributed to the discrepancy.
Documentation of Controlled Substance Discrepancy Resolution
- If the discrepancy is active in the Cubex system, document the resolution in the notes section when resolving the discrepancy. The notes should include sufficient detail to determine what was decided. As a rule, someone not involved in resolving the discrepancy should be able to review the resolution and be able to recreate the reasoning behind it.
- If a prior transaction was incorrect, the transaction details should be provided and the resolution (e.g., corrected in HIS, etc.) should be provided in sufficient detail to support auditing.
- Hub loss as the resolution should include a number of sticks, the date those sticks were counted back to, and the calculated hub loss allowed. If the hub loss is recalculated with the 0.06ml/stick method, then the calculated volumes for both 0.05ml/stick and 0.06ml/stick should be indicated as well as the word ‘WATCHING’ in all caps.
- If the discrepancy is associated with a drug outside of the Cubex system, document resolution notes next to the transaction log indicating the cycle count/actual draw. If additional space is needed, indicate that notes are continued on the back or attached additional sheets.
- Resolution details should include the same amount of detail as Cubex discrepancy resolutions.
- For discrepancy resolution that cannot be documented by either of the methods described above (e.g., a correct to a prior Cubex discrepancy resolution), document the discrepancy resolution in the Discrepancy Resolution Spreadsheet with the same level of detail used for all other discrepancies.
Auditing Controlled Substance Discrepancy Resolutions
- The VTH Controlled Substance Surveillance Program Manager will perform a monthly audit of resolved Cubex controlled substance discrepancies. The purpose of this audit is to ensure that Cubex discrepancies are being resolved accurately and documented with sufficient detail to identify potential issues.
- The audit should involve the following steps:
- Generate a Cubex report of all resolved discrepancies over the previous month.
- Focusing on the controlled substances, review the notes from each resolution. Flag any discrepancy resolutions that seem inappropriate or unusual.
- Investigate each flagged discrepancy to determine if the resolution was correct and documented sufficiently.
- For any incorrect resolutions, determine the appropriate resolution and document on the Discrepancy Resolution Spreadsheet.
- Provide the VTH DEA Registrant with the results of the monthly review and any concerns or trends identified.
Definition(s):
- Automated Dispensing Cabinet (ADC): The drug dispensing cabinet used within the VTH. (E.g., Cubex and Omnicell).
- Controlled Substance: A drug or other substance, or immediate precursor, included in schedule I, II, III, IV, or V of part B of Title 21 USC Controlled Substances Act. This term does not include distilled spirits, wine, malt beverages, or tobacco.
- DEA Registrant: The DEA license holder under whom controlled substances are purchased by the Medication Dispensary for VTH use.
- Discrepancy: A difference in the actual amount of drug on hand compared to the amount expected based on transaction logs.
- Discrepancy Resolution Spreadsheet: An Excel document for tracking controlled substance discrepancies and their eventual resolution when documentation on the transaction log (paper log or ADC log) is not feasible. The document is a required controlled substance record and as such, must be maintained for a minimum of five years.
- Health Information System (HIS): The computer system used to maintain electronic patient records and statements.
- Medication Dispensary Coordinator: Supports the Pharmacy Service Head in overseeing day-to-day Medication Dispensary operations, ensuring compliance with all applicable laws and institutional policies. This role may also include serving as the VTH Controlled Substance Surveillance Program Manager.
- Pharmacy Service Head: A licensed pharmacist serving as the Pharmacist in Charge (PIC), who oversees all Medication Dispensary operations, working in conjunction with the Medication Dispensary Coordinator to ensure legal, regulatory, and institutional requirements are fully met.
- Transaction Logs: Documentation pertaining to controlled substance access, administration, and wasting. This includes but is not limited to Automated Dispensing Cabinet (ADC) logs, paper logs, and patient charts.
- Veterinary Teaching Hospital (VTH): The collective clinical services of the Large Animal Clinic, Midwest Equine, the Small Animal Clinic, and the Veterinary Medicine South Clinic. This does not include the Medical District Veterinary Clinic (MDVC).
- VTH Controlled Substance Surveillance Program Manager: The person working within the Veterinary Teaching Hospital (VTH) who is responsible for implementing the oversight program for all clinical controlled substance activities, including students on rotation.
Applicable Regulations:
Federal
- 1301.71 (a) Security requirements generally: "All applicants and registrants shall provide effective controls and procedures to guard against theft and diversion of controlled substances…"
- 1301.76 (b) Other security controls for practitioners: “The registrant shall notify the Field Division Office of the Administration in his area, in writing, of the theft or significant loss of any controlled substances within one business day of discovery of such loss or theft. The registrant shall also complete, and submit to the Field Division Office in his area, DEA Form 106 regarding the loss or theft…”
- 1301.91 Employee responsibility to report drug diversion: “…an employee who has knowledge of drug diversion from his employer by a fellow employee has an obligation to report such information to a responsible security official of the employer… A failure to report information of drug diversion will be considered in determining the feasibility of continuing to allow an employee to work in a drug security area.”
- 1301.92 Illicit activities by employees: “…employees who possess, sell, use or divert controlled substances will subject themselves not only to State or Federal prosecution for any illicit activity, but shall also immediately become the subject of independent action regarding their continue employment…”
- 1304.04 (a) Recordkeeping duration: "…every inventory and other records required to be kept under this part must be kept by the registrant and be available, for at least 2 years from the date of such inventory or records, for inspection and copying by authorized employees of the Administration."
- 1304.21 (a, b, e) General recordkeeping requirements: "...Shall maintain, on a current basis, a complete and accurate record of each substance manufactured, imported, received, sold, delivered, exported, or otherwise disposed of by him/her…Separate records shall be maintained by a registrant for each registered location...In addition to any other recordkeeping requirements, any registered person that destroys a controlled substance...shall maintain a record of destruction on a DEA Form 41. The records shall be complete and accurate and include the name and signature of the two employees who witnessed the destruction. Except, the destruction of a controlled substance dispensed by a practitioner for immediate administration at the practitioner's registered location, when the substance is not full exhausted, shall be properly recorded...and such record need not be maintained on a DEA Form 41."
- 1304.22 (a2i,ii,iv,vii,ix) (c ) Required records: "…For each controlled substance in finished form, the name of the substance, each finished form and the number of units or volume of finished form in each commercial container, the number of units of finished forms and/or commercial containers acquired from other persons, including the date of and number of units and/or commercial containers in each acquisition to inventory and the name, address, and registration number of the person from whom the units were acquired, the number of commercial containers distributed to other persons, including the date of and number of containers in each reduction from inventory, and the name, address, and registration number of the person to whom the containers were distributed, the number of units of finished forms and/or commercial containers distributed or disposed of in any other manner by the registrant, including the date and manner of distribution or disposal, the name, address, and registration number of the person to whom distributed, and the quantity in finished form distributed or disposed...the number of units or volume of such finished form disposed, including the name and address of the person to whom it was dispensed, the date of dispensing, the number of units or volume disposed, and the written or typewritten name or initials of the individual who dispensed or administered the substance on behalf of the dispenser.”
State
- 720 ILCS 570/201 (h) Security requirements generally: "Persons registered with the Drug Enforcement Administration to manufacture or distribute controlled substances shall maintain adequate security and provide effective controls and procedures to guard against theft and diversion…"
- 3100.310 (a) Security requirements generally: “All applicants and licensees shall provide effective controls and procedures to guard against theft and diversion of controlled substances…”
- 3100.360 (d) Record and inventorying requirements generally: “After a loss or theft of controlled substances, a licensee shall conduct an approximate count inventory with a start date of the last inventory for the controlled substance that was either lost or stolen.”
- 3100.360 (f) Record and inventorying requirements duration: “Every licensee shall keep a suitable book, file or electronic record keeping system in which shall be preserved for a period of not less than 5 years, [all prescription dispensing information].”