Microanalysis - TA Instruments Affinity ITC (web)
Client Operated Instrument
The TA Affinity isothermal titration calorimeter (ITC) can be used to determine the thermodynamic properties of a chemical or physical equilibria in solution. Binding affinity (Kd), enthalpy changes (ΔH), and binding stoichiometry (n) of the interaction between two or more molecules in aqueous solution can be determined quantitatively. Gibbs free energy (ΔG) and entropy changes (ΔS) can also be characterized. Users who wish to be trained should contact the lab to schedule training. Instrument time is reserved through ChemFOM.

Key Features and Specifications:
- Active cell volume: 190 µL
- Maximum cell volume: 550 µL
- Cell composition: gold
- Stirring speed range: 0-250 rpm
- Injection volume: 1-3 µL
- Operating temperature: 2 to 80°C
- Baseline stability: 0.02 µWatt/hr
- Minimum detectable heat: 0.04 µJ
- Maximum detectable heat: 5,000 µJ
- Reference cell volume: 300 µL
Theory of Operation
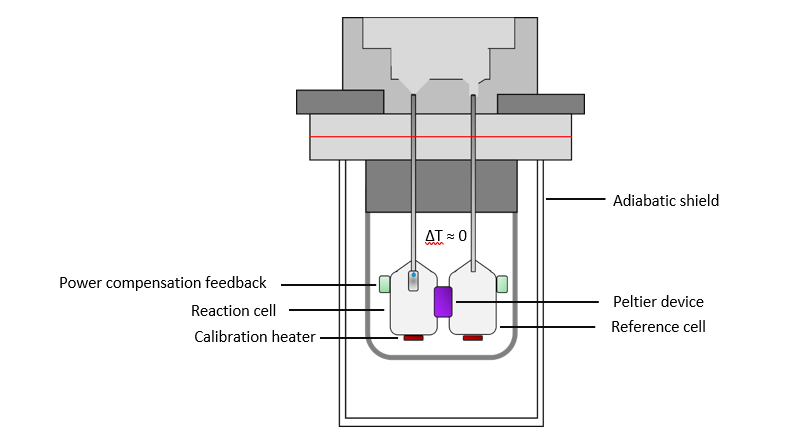
A typical ITC instrument consists of a reference cell and a reaction cell, made of a highly conductive material, such as gold. The reference cell is filled with water as it behaves similar to most buffer solutions and allows for easy reference cell maintenance. The cells are located in an adiabatic jacket and are connected by a thermoelectric device, such as a Peltier device that is sensitive to relative changes in the cells’ temperatures and is connected to a feedback power supply. The instrument applies or withdraws power to keep the change in temperature, ΔT, between the reference and sample cell constant while performing an experiment. The pressure is also held constant during the experiment. When heat is given of in an exothermic reaction, the heater decreases its power to the cell to maintain a constant temperature. In the case of an endothermic event, the heater will increase its power to maintain a constant ΔT. Observations are plotted as the power needed to maintain the reference and the sample cell at an identical temperature against time. Therefore, the experimental raw data consists of a series of spikes of heat flow (power), with every spike corresponding to an injection. These heat flow spikes/pulses are integrated with respect to time, giving the total heat exchanged per injection.
Link to manufacturer's brochure: https://www.tainstruments.com/pdf/brochure/BROCH-MICRO-EN.pdf